Glossary of Chemical Classes
Alcohols are organic compounds of the general formula ROH, where R may be aliphatic, alicyclic, or include aromatic rings. Alcohols may be also classified by the position of the hydroxyl group on the aliphatic carbon chain as primary, secondary or tertiary; or by the number of the hydroxyl groups, for example as diols or triols.
Aldehydes are organic compounds containing one or more HC=O groups. Aldehydes may be aliphatic, alicyclic, heterocyclic or aromatic.
Alkanolamides are the condensation products of Fatty Acids with primary or secondary Alkanolamines. They conform generally to the formula R(C=O)NXY, where R(C=O) is a fatty acid radical; and X represents the radical -CHR'CH2OH, in which R' may be H or lower alkyl. The substituent Y may be the same as X, may be H (in the case of primary alkanolamines) or an alkyl group, alkanol group, Alkanolamides are used as surfactants and occasionally as emulsifiers.
Alkanolamines are substances which carry aliphatic hydroxyl and amino groups on the same molecules. The presence of the hydroxyl group reduces the volatility of these amines. Mono-, di-, and triethanolamines are typical representatives of this type of substance. Because they are used so commonly, they are generally referred to by their abbreviations: MEA, DEA, TEA, etc
Alkenes are hydrocarbons that have a carbon-carbon double bond. They generally conform to the formula CnH2n.
Alkoxylated Alcohols are Ethers formed from the reaction of an Alcohol with an alkylene oxide, generally ethylene oxide or propylene oxide. Because the ether formed from the reaction of one molecule of an alcohol with one molecule of the alkylene oxide is also an alcohol, the reaction with the alkylene oxide can continue until the latter is consumed. The original alcohol may be aliphatic, heterocyclic, or aromatic (Phenol), and the alkylene oxide may carry various substituents, R', e.g., H, CH3, and the like. Mixtures of alkylene oxides may also be used in the alkoxylation reaction. In addition, n can vary widely. As a result, a large number of alkoxylated alcohols of widely different character can be synthesized. They are useful as surfactants, emulsifiers, solubilizers, and conditioners. Their use is particularly attractive in light of their chemical inertness to hydrolytic decomposition. Compounds identified by the names laneth, laureth or octoxynol are typical representatives of this group of materials.
Alkoxylated Amines are the alkoxylation products of primary and secondary amines and their salts. In contrast to the Alkoxylated Amides, these amines are slightly basic and can be further reacted to yield tertiary amines and Quaternary Ammonium Compounds and their salts. If the alkoxylation is conducted on a compound containing more than one reactive hydrogen atom (an H atom attached to an O or N atom), the structure of the end product may be complex or even polymeric.
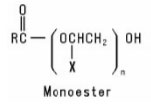
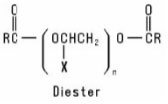
Alkoxylated Carboxylic Acids are formed when a Carboxylic Acid is reacted with an alkylene oxide or with a preformed Polymeric Ether. The resulting product may be a monoester or a diester or a mixture of the two, depending on the reaction conditions. Their general structures are:
Alkyl Aryl Sulfonates are aromatic sulfonic acids (or their salts) in which the aromatic ring carries an alkyl chain. They are commonly produced by sulfonation of an alkylbenzene or similar aromatic hydrocarbon; the resulting strong organic acid can then be neutralized with a variety of bases, such as sodium hydroxide. Long-chain linear alkyl groups are preferred in modern detergent usage, since they are more biodegradable than the branched-chain derivatives. Short-chain alkyl aryl sulfonates are used (as hydrotropes) in detergent-containing products in order to increase the solubility of other surfactants and to control viscosity.
Alkyl Aryl Ether Sulfates are the salts of the sulfuric acid monoesters of alkoxylated alcohols, with the aromatic ring carrying an alkyl chain. They are prepared by sulfation of corresponding ethoxylated alcohols and subsequent neutralization with a suitable base, e.g., triethanolamine. They have the structure R(OCH2CH2) nOSO3-M+. R represents the alkyl chain derived from either natural or synthetic sources; n, the degree of ethoxylation, normally ranges from one to four but can be higher; M+ identifies an alkali metal or Amine.
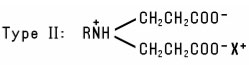
Alkyl-Substituted Amino Acids (including salts and alkyl-substituted imino acids; excluding betaines) include several types. One group of Alkyl-Substituted Amino Acids is prepared by reaction of acrylic acid with fatty Amines. The resulting products conform to the Type I or Type II structure:

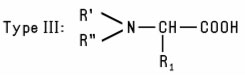
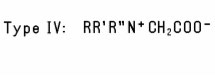
e.g., Disodium Tallowiminodipropionate. Another group of Alkyl-Substituted Amino Acids (Type III) is formed when the N-atom in a natural α-Amino Acid is alkylated to form a mono- or disubstituted derivative such as Dimethyl Aspartic Acid. A fourth type of Alkyl-Substituted Amino Acids is formed when a dialkyl amino acid is further alkylated with a third alkyl substituent (Type IV). A similar type of compound is formed when an alkyl-substituted tertiary amine is reacted with chloroacetic acid. R' and R'' are frequently identical (sometimes OH-substituted) short-chain alkyl groups, as in the case of Dihydroxyethyl Soy Glycinate. The N-atom in Type IV derivatives always carries a positive charge, regardless of pH. Like simple amino acids, the four types of alkyl-substituted amino acids may exist in the form of zwitterions, depending on pH. Type IV Alkyl-Substituted Amino Acids in which the N-atom carries two methyl groups are listed separately as Betaines. These substituted amino and imino acids are commonly used as amphoteric emulsifiers and detergents, as foaming agents, and as skin and hair conditioning agents.
Alkyl Sulfates are the salts of the sulfuric acid monoesters of Fatty Alcohols. They are prepared by sulfation of the alcohols and subsequent neutralization with a suitable base, e.g., ammonia. They have the structure ROSO3+M+, where R represents the alkyl chain of the fatty alcohol and M+ the alkali metal or other cation. Alkyl Sulfates are Esters and, therefore, subject to hydrolysis, especially at extreme pHs. A typical example of this class is Sodium Lauryl Sulfate. They are also frequently employed as auxiliary emulsifiers and solubilizers.
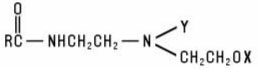
Alkylamido Alkylamines are the group of amphoteric materials that exhibit the structure:
Alkynes are hydrocarbons that have a carbon-carbon triple bond. They generally conform to the formula CnH2n-2.
Amides (including salts; excluding alkanolamides and alkoxylated amides) are compounds which exhibit the structure RCO-NXY, where RCO- commonly denotes a Carboxylic Acid or a Fatty Acid radical. X and Y may be H or any alkyl or aromatic radical; X and Y may be the same or different. The amide structure may be part of a heterocyclic ring, as in PCA. R can also represent a nitrogen atom, as in Urea. Amides are hydrolyzable derivatives of carboxylic acids and are prepared via a number of synthetic and biological routes. A typical representative includes Cocamide. Alkanolamides are a special group of amides in which the amide nitrogen carries a hydroxyl-substituted alkyl group. Amides and Alkanolamides can be further reacted with alkylene oxides to form Alkoxylated Amides. Acylated amino acids are included in the listing of Amino Acids. Amides have a number of applications, which include use as preservatives, surfactants, and foam boosters.
Amine Oxides are derived from tertiary Amines by oxidation, usually with hydrogen peroxide. They conform to the general structure, RR'R''NO, where R represents a long-chain alkyl group and where R' and R'' may be either identical or different and represent relatively short-chain alkyl groups. A typical example is Lauramine Oxide, which chemically is N,N-dimethyllauramine oxide. The Amine Oxides are nonionic surfactants with mildly cationic properties at acid pH. They are used in a variety of cleaning products and as emulsifiers.
Amines (including amine salts and excluding alkanolamines) are the organic substitution compounds of ammonia, NH3. In primary amines the amino group is singly substituted, i.e., RNH2; in secondary amines, the amino group carries two organic substituents, i.e. RR'NH; in tertiary amines, three organic radicals are attached to the amino nitrogen atom, i.e., RR'R''N. If the nitrogen carries four substituents, the compounds are Quaternary Ammonium Compounds. In secondary or tertiary amines the substituents may be the same or different. Two or more amino groups may be found on the same molecule, e.g., in diamines. The organic substituents in amines may be aliphatic, aromatic, or heterocyclic and may carry other functional groups. Amino Acids result if the additional substituent on the amine is a Carboxylic Acid grouping. Amides and Alkanolamides are formed from primary or secondary amines by reaction with a reactive species of a carboxylic acid.
Amino Acids (including salts, esters and acyl derivatives; excluding alkyl substituted amino acids and protein derivatives) are compounds carrying both amino groups (see Amines) and Carboxylic Acid groups on the same molecule. In modern usage, the term "amino acid" is sometimes (erroneously) restricted to the principal products of the hydrolysis of Proteins. In this sense, amino acids are the monomeric building blocks of peptides and proteins. Most of the amino acids from natural sources, the so-called alpha-amino acids, may be represented by the general structure RCH(NH2)COOH, in which R represents an aliphatic, aromatic, or heterocyclic grouping. In general, the natural amino acids, with the exception of Glycine, possess one or more asymmetric centers and, therefore, may exist in at least two optically active forms. Most of the natural amino acids are of the L-configuration; however, a number of D-amino acids have been found in bacterial cell wall hydrolysates or in some fungal antibiotics. Since amino acids carry two types of salt-forming functions, their behavior in aqueous solutions is dependent on pH. At low pH, the amino group is protonated, and amino acids act as cations. At high pH, the carboxyl groups are ionized, and these acids behave as anions. At intermediate pHs, i.e., near neutrality, the amino group may partially neutralize the carboxyl group. In this case, the amino acid exists as a zwitterionic molecule possessing both cationic and anionic charges on the same molecule. The carboxylic acid groups of amino acids can be esterified, as, e.g., in Ethyl Aspartate. Alternately, the amino group can be acylated with a Fatty Acid, as, e.g., in Sodium Cocoyl Glutamate. Amino acids derived from the hydrolysis of various proteins are used as conditioning and specialty ingredients. Other commonly used amphoteric amino acids include the Alkylamido Alkylamines, a variety of AlkylSubstituted Amino Acids, and Betaines.
Anthraquinones are derivatives of 9,10-anthraquinone or 9,10-dioxoanthracene - an aromatic compound C14H8O2, wherein the keto groups are located on the central ring.
Azo Compounds are derivatives of diazene (diimide), HN=NH, wherein both hydrogens are replaced by aryl or alkyl substituents.
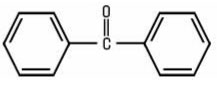
Benzophenones constitute a group of aromatic Ketones, all of which contain the diphenylketone structural element:
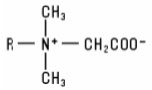
Betaines (including “sultaines”) The Betaine group of raw materials includes the quaternized alkyl or substituted alkyl derivatives of N,N-dimethyl glycine. The Betaines have the structure:
Biological Polymers and their Derivatives (Including salts, excluding gums, hydrophilic colloids and derivatives) do not form a chemically homogeneous class except for the fact that they are built up from naturally occurring monomers frequently of the Carbohydrate type. Included are materials obtained from edible grains, and animal products. Biological polymers can be used as originally obtained from their source, e.g., Zea Mays (Corn) Starch, or may be chemically modified to alter solubility and swelling characteristics, e.g., Glyceryl Starch.
Biological Products are a large and diverse class of materials not defined chemically. Specific ingredients derived from biological sources are classified on the basis of their chemistry and are not included in this listing. The majority of the materials in this class are mixtures derived from plants (herbs, roots, flowers, fruits, or seeds), but some animal-derived materials are included. Lanolin and Lanolin Derivatives are described as a separate chemical class, as are the plant and animal-derived Proteins, Protein Derivatives, Carbohydrates, and Biological Polymers. Other biological ingredients are classified as Fats and Oils or Essential Oils and Waters.
Carbohydrates (monosaccharides, disaccharides, polysaccharides, glycosaminoglycans and derivatives; excluding gums) were originally thought of as the hydrates of carbon, but they are more adequately defined as polyhydroxy- Aldehydes and Ketones and their derivatives. They normally conform to the general structure CX(H2O)y. The term carbohydrates includes all the sugars, starches, celluloses, and a wide variety of Gums, and Biological Polymers. This broad class of compounds is further subdivided into monosaccharides, disaccharides, and polysaccharides. Monosaccharides or simple sugars are represented by the formula: CH2OH(CHOH)nCHO if the compound is an aldehyde derivative, i.e., an aldose; or CH2OH(CHOH)nCOCH2OH if the compound is a ketone, i.e., a ketose. The major 6-carbon monosaccharides are Glucose (an aldose in which n equals 4), Fructose (a ketose in which n equals 3), and Galactose, which is chemically similar to Glucose but possesses a different steric configuration. Disaccharides are Ethers, formed from two monosaccharides. The most common disaccharides are Maltose (two glucose units), Sucrose (one glucose and one fructose unit), and Lactose (one glucose and one galactose unit). Polysaccharides are the polyethers of monosaccharides which generally occur in nature. They may be polymers of the same monosaccharide, in which case they are known as homopolysaccharides; or may include differing and occasionally carboxyl-substituted monosaccharides, in which case they are known as heteropolysaccharides. Starch is a homopolysaccharide derived from Glucose; Cellulose is also built up from glucose units but differs from starch in the configuration around the ether oxygen bonds. Heteropolysaccharides found in terrestrial and oceanic plants are important gums and natural polymers. Polysaccharides found in the animal kingdom include Chitin and a large group of complex polysaccharides which are chemically bound to proteins, e.g., glycosaminoglycans (mucopolysaccharides), Hyaluronic Acid and Chondroitin Sulfate. All types of carbohydrates are used in finished products to impart viscosity or other textural characteristics.
Carboxylic Acids are a group of synthetic or naturally occurring organic acids which contain at least one -COOH group. Carboxylic Acids can have widely differing properties. Most of them are weak acids, some are fairly strong acids, e.g., Citric Acid; some are readily water soluble, e.g., Acetic Acid; some are classified as vitamins, e.g., Folic Acid. Carboxylic Acids can form salts with almost all cations. The one unifying feature of this group is the -COOH (carboxyl) function. Carboxylic Acids find a diverse range of applications. In addition, they are used in the synthesis of other ingredients (see Esters, Fats and Oils, and Glyceryl Esters). Fatty acids are included separately in the list of Fatty Acids. Salts of Carboxylic Acids are listed under Organic Salts.
CAS Registry Number is a unique numerical identifier assigned by the Chemical Abstracts Service. These numbers are assigned to every chemical described in the open scientific literature, which include elements, isotopes, organic and inorganic compounds, ions, organometallics, metals, and nonstructurable materials. The CAS Numbers are assigned in sequential, increasing order when the substance is identified by CAS scientists for inclusion in the database, they are not related to chemistry and have no inherent meaning. A CAS Registry Number is separated by hyphens into three parts, the first consisting of up to seven digits, the second consisting of two digits, and the third consisting of a single digit serving as a check digit. The CAS numbers serve as reliable, common, and internationally recognized system to identify specific substances used by branches of science, industry, and regulatory bodies. For more information, click here.
EPA’s Safer Choice Standard (formerly, the ‘DfE Standard for Safer Products’)) works in partnership with industry, environmental groups, and academia to reduce risk to people and the environment by finding ways to prevent pollution. EPA’s Safer Choice Standard requires ingredient communication and references the CSPA Consumer Product Ingredients Dictionary as an acceptable source of nomenclature to satisfy the EPA’s Safer Choice Standard ingredient disclosure provisions. For more information, click here.
EPA List of Ingredients Eligible for FIFRA 25(b) Pesticide Products is the list of minimal risk ingredients that are eligible for inclusion in pesticide products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA)’s Section 25(b). For more information, click here.
EPA Safer Chemical Ingredients List for Use in DfE-Labeled Products contains chemicals that meet the criteria of the Design for the Environment (DfE) Safer Product Labeling Program. This voluntary program recognizes products that are high-performance and cost-effective using the safest chemical ingredients. This list of safer chemical ingredients is arranged by functional-use class and will assist product manufacturers in identifying chemicals that the DfE program has already evaluated and identified as safer. For more information, click here.
Essential Oils and Waters are a diverse group of materials derived from a wide variety of plant materials. They are prepared by a number of processes including, but not limited to, steam or dry distillation, flash pasteurization and mechanical processes such as cold-pressing. Cold-pressed essential oils are usually prepared from the peels of citrus fruits. Such processes reportedly, do not chemically alter the ingredients from those found naturally in the whole plant. Essential Oils and Waters are prepared from the leaves, stems, flowers, bark, roots, or other parts of a plant or the whole plant. The most widely used method for preparing Essential Oils from plants is associated with steam distillation. The condensate from steam distillation produces two distinct fractions that contain the volatile ingredients from the plant. The water insoluble fraction contains the "oil." The water soluble fraction contains ingredients from the plant that are water soluble. The name assigned to the water insoluble fraction from steam distilled plant materials is identified by the term "Oil" in the INCI name. The water soluble fraction from the steam distilled plant material will be identified by the term "Water" in the CSPA Name. Different names are assigned to "Waters" that are prepared by steam distillation from those that are prepared by adding water to materials isolated by solvent extraction of plant materials, i.e., extracts, or other by processes. The latter are named as mixtures. For example, if a plant material is prepared by the addition of water to a plant extract the CSPA Name would include the name of the extract followed by the term water. In addition to Essential Oils and Waters there are a number of other types of plant derived preparations. They include, e.g., extracts, juices, gels, saps, tars, gums and powders to name a few. Extracts may include such preparations as tinctures, concretes, resinsoids, or absolutes. Extracts are the largest group of plant derived ingredients. The term "essential" in the name "essential oil" is a term of art and is a misnomer in that these oils are not "essential" as that term is often related to a material being "indispensable." It appears that this term was applied to indicate that the oil represents the characteristics associated with the properties of odor or flavor of the plant. The term "oil" is also a misnomer in that essential oils do not have an oily-feeling or have the properties of conventional oils, i.e., the so-called fixed oils that are referred to as fats and oils commonly found in plant and animal tissues such as Coconut Oil or Shark Liver Oil.
Esters are covalent compounds formed between acids and Alcohols. Esters can be formed from almost all acids (inorganic and carboxylic) and any alcohol. Thus, the number of naturally occurring and synthetically prepared esters used is very large. In order to subdivide the list of esters, most have been assigned to other chemical classes on the basis of some additional chemical features: Alkoxylated Fatty Acids, Alkyl Ether Sulfates, Alkyl Sulfates, Fats and Oils, Glyceryl Esters, Phosphorus Compounds, Isethionates, Sorbitan Derivatives, Sulfosuccinates, Sulfuric Acid Derivatives, and Waxes. Esters belonging to this specific class are derived from carboxylic acids and have the structure RCOOR' where RCO- represents the carboxylic acid radical and where -OR' is the alcohol residue. Included are low and medium molecular weight compounds, e.g., Butyl Acetate or Isopropyl Myristate; some polymers, e.g., Polyvinyl Acetate. They are subject to hydrolysis into alcohols and acids at extreme pHs and at elevated temperatures.
Ethers are the organic compounds possessing a C-O-C system of bonds. If this C-O-C system is part of a ring, the corresponding compound is classified with the Heterocyclics, e.g., Tocopherol. Sorbitan Derivatives are also heterocyclic ethers. Most ethers are prepared synthetically. Alkoxylated derivatives are ethers, but are herein classed as Alkoxylated Alcohols, Alkoxylated Amides, Alkoxylated Amines, Alkoxylated Carboxylic Acids, and Polymeric Ethers. The esterified polyglyceryl ethers resulting from the dehydration of glycerin are included in the group of Glyceryl Esters. Ethers are primarily used as solvents or as fragrance or flavor components.
Exempted VOC (exempted volatile organic compound) refers to the status of the ingredient in the U.S. Environment Protection Agency National Volatile Organic Compound Emission Standards for Consumer Products (i.e., the “National Consumer Products Rule”) that is codified at 40 C.F.R. Part 59 Subpart C, as well as the California Air Resources Board “Consumer Products Regulation” that is codified at Cal. Code Regs. Title 17 §§ 94507-17, and numerous state air quality regulations based on the Ozone Transport Commission Mode Rule for Consumer Products. In some cases, however, the adoption of VOC exemptions by states (especially California) may lag behind the adoption of the exemption by U.S. EPA, so current individual state regulations should always be consulted for compliance determinations.
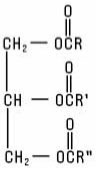
Fats and Oils are the glyceryl esters of Fatty Acids (triglycerides) normally found in animal and plant tissues, including those which have been hydrogenated to reduce or eliminate unsaturation. Also included are synthetically-prepared esters of glycerin and fatty acids. They possess the structure:
Fatty Acids are the Carboxylic Acids obtained by hydrolysis of animal or vegetable Fats and Oils. Carboxylic acids having alkyl chains shorter than about seven carbon atoms are not commonly considered fatty acids. Natural fatty acids may be saturated or unsaturated and generally contain an even number of carbon atoms. Fatty Acids have the general structure R-COOH. A few chemically synthesized substances (which are rarely found in nature) are included in the listing of fatty acids. Fatty acids may be saturated or unsaturated. Palmitic Acid is a typical saturated fatty acid; Oleic Acid is a monounsaturated acid, i.e., contains one cis-double bond. The double bonds in naturally available fatty acids are almost always cis and are not conjugated. Docosahexaenoic acid is a polyunsaturated C22 fatty acid containing six double bonds. Fatty acids derived from natural triglycerides, e.g., Corn Acid, are blends of saturated and unsaturated fatty acids. Fatty acids are a common source of raw materials for the synthesis of ingredients. They are used in the form of their salts, i.e., as Soaps, which are frequently formed in situ from the acid and a suitable alkali. These salts are emulsifiers and suspending agents and possess foaming characteristics which are desirable in shaving preparations and can be used in cleaning products. Free fatty acids are used to produce superfatted soaps and as opacifiers and thickening agents.
Fatty Alcohols are higher molecular weight nonvolatile alcohols. They are produced from natural Fats and Oils by reduction of the Fatty Acid -COOH grouping to the hydroxyl function. Alternately, several completely synthetic routes yield fatty alcohols which may be structurally identical or similar to the nature-derived alcohols. Fatty Alcohols generally are primary alcohols conforming to the structure RCH2OH; those fatty alcohols prepared from naturally occurring fatty acids normally contain an even number of carbon atoms.
Glyceryl Esters and Derivatives comprise a subgroup of Esters which are primarily Fatty Acid mono- and diglycerides or triglycerides modified by reaction with other alcohols and the like.
Glycol Ethers are a group of solvents based on alkyl ethers of ethylene or propylene glycol. Glycol ethers can be also derived of diethylene or dipropylene glycol, and therefore contain two or more ether groups.
Glycols are chemical compounds containing two hydroxyl groups (-OH groups). Examples of simple glycols are ethylene glycol and propylene glycol. Compounds with three or more hydroxyl groups are call Polyols.
Gums, Hydrophilic Colloids and Derivatives are generally plant-derived materials which belong to the chemical class of Carbohydrates. Although chemically diverse, the unique ability of gums to swell in the presence of water and to increase the viscosity of aqueous preparations accounts for this special class. The viscosity developed by hydrophilic colloids depends on their molecular weight and the presence of various cations which may neutralize some acid functions of these carbohydrate molecules or cause some cross linking. Gums and the like are used to impart viscosity to some types of products. They act as suspending or gelling agents and emulsion stabilizers. Some of these gums have unique textural qualities which make them useful in water-based lubricants.
Halogen Compounds are organic chemicals which contain one or more halogen atom(s) (Cl, Br, I, or F) covalently bonded to a carbon atom. Halogen Compounds find uses as solvents, preservatives, colorants and propellants.
Heterocyclic Compounds (including salts; excluding polymers and imidazolines) are aromatic or alicyclic compounds in which the ring contains one or more atoms other than carbon. The most common elements found in heterocyclics are nitrogen (e.g., PCA, Zinc Pyrithione, or Pyridoxine), oxygen (e.g., Furfural or Dimethyl Isosorbide), or sulfur (e.g., Dibenzothiophene). Multiple replacement of carbon in rings is not uncommon, as in Methylchloroisothiazolinone [N+S], Guanosine [4N+O], or Morpholine [N+O]. .
Hydrocarbons are the group of compounds containing only carbon and hydrogen. Hydrocarbons are generally derived from petrochemicals, but some of them are found in the plant and animal kingdom (e.g., d-Limonene). Their structures can vary widely, and include aliphatic, alicyclic, and aromatic compounds. This category also includes some materials of complex composition which can be a source of a variety of hydrocarbons. Typical are petrolatum, paraffin, and mineral oil, all of which are widely used because of their nonvolatility and unctuous character. Lower molecular weight volatile hydrocarbons are used as solvents (e.g., Mineral Spirits). The hydrocarbons which are normally gases at room temperature (e.g., Butane or Propane) are used as aerosol propellants. Finally, it should be noted that many Synthetic Polymers are hydrocarbons (e.g., Polyethylene).
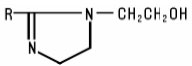
Imidazoline Compounds are substituted imidazolines including compounds having the following generic structure:
Inorganic Acids are acids which contain no organic carbon atoms. With some notable exceptions, such as boric acid, they are strong acids, i.e., they are largely ionized into protons and the corresponding anion in aqueous solution. They are often used to neutralize bases (by forming Inorganic Salts or Organic Salts), to adjust finished products to the desired hydrogen ion concentration (pH), and are occasionally employed to form buffering systems. Inorganic Acids are sometimes called mineral acids.
Inorganic Compound (inorganic compound) refers to the status of the ingredient in the U.S. Environment Protection Agency National Volatile Organic Compound Emission Standards for Consumer Products (i.e., the “National Consumer Products Rule”) that is codified at 40 C.F.R. Part 59 Subpart C, as well as the California Air Resources Board “Consumer Products Regulation” that is codified at Cal. Code Regs. Title 17 §§ 94507-17, and numerous state air quality regulations based on the Ozone Transport Commission Mode Rule for Consumer Products. Inorganic compounds are not considered to be VOCs or LVP-VOCs.
INCI (The International Nomenclature of Cosmetic Ingredients) is a system of names for chemicals and other ingredients of cosmetic products. The INCI system evolved since 1940s as a response to the problem in identifying and describing common cosmetic ingredients faced by the cosmetic industry. To address this problem, first a Board of Standards was instituted by the Toilet Goods Association, which later changed names to CTFA (Cosmetic, Toiletry, and Fragrance Association), and eventually to PCPC (Personal Care Products Council). The PCPC works to develop specifications and descriptions for common ingredients and sponsors annual scientific meetings to address specific issues of importance to cosmetic chemists. INCI labeling system is recognized and used worldwide and contains tens of thousands of ingredients used in personal care products. For more information, click here.
Inorganic Bases are alkalies which contain no organic carbon atoms. Carbonates of alkaline earths and alkali metals are included in the listing of Inorganic Salts. In water, inorganic bases may be strongly or weakly ionized into hydroxyl ions and corresponding cations. Not all inorganic bases are soluble in water. They are used to neutralize acids (by forming Inorganic Salts or Organic Salts), to adjust finished products to the desired hydrogen ion concentration (pH), and are occasionally employed to form buffering systems.
Inorganic Salts are the compounds formed when an inorganic base reacts with an inorganic acid. Under these circumstances, the base provides the cation while the anion is derived from the acid. In the naming of Inorganic Salts, the name of the cation (or cations) normally precedes the name of the anion (e.g., Sodium Sulfate or Aluminum Zirconium Octachlorohydrate). Salts as a rule are ionized and, in the case of water-soluble salts, cations and anions are present as individual species although electroneutrality must be maintained. Thus an aqueous solution of silver nitrate contains silver cations and nitrate anions in stoichiometrically equivalent amounts. Solubility in water is not a criterion for ionization since solids (e.g., Sodium Chloride) have been shown to be ionized in their crystalline forms. Salts are used for diverse purposes, which include thickening, buffering, freeze-point depression, and as oxidizing or reducing agents.
Inorganics (including minerals and oxides) include mined materials, various inorganic gases, liquids, or solids, some elemental substances, and inorganic oxides. Excluded are all substances which are classified as Inorganic Salts, Inorganic Acids or Inorganic Bases. As a rule, inorganics are chemically inert under conditions of use. Some of them are used as pigments, while others are fillers, abrasives and smoothing agents. The gaseous materials are used as propellants (e.g., Nitrogen). Clay-type materials) are used to increase the viscosity of aqueous suspensions. Hydrogen Peroxide is an important oxidizing agent which is used for bleaching.
Isocyanates are organic compounds that contain the isocyanate functional group N=C=O and generally conform to the formula: R−N=C=O.
Ketones may be viewed as the oxidation products of secondary Alcohols. Their structure R-CO-R' is reminiscent of that of Aldehydes (in which R' is hydrogen). R and R' may be the same or different and may be aliphatic, aromatic, or alicyclic. As a group, ketones are stable to hydrolytic reactions and oxidation. The best known ketone, Acetone, and other low molecular weight ketones are used as solvents. Some ketones are used as fragrance materials and for various specialty purposes.
LVP-VOC (low-vapor-pressure organic compound) refers to the status of the ingredient in the U.S. Environment Protection Agency National Volatile Organic Compound Emission Standards for Consumer Products (i.e., the “National Consumer Products Rule”) that is codified at 40 C.F.R. Part 59 Subpart C, as well as the California Air Resources Board “Consumer Products Regulation” that is codified at Cal. Code Regs. Title 17 §§ 94507-17, and numerous state air quality regulations based on the Ozone Transport Commission Mode Rule for Consumer Products. As used in this Dictionary, refers to organic compounds with measurable vapor pressure below 0.1 mm Hg at 20 degrees C.
Nitriles are organic compounds that contain the −C≡N functional group and generally conform to the formula: R−C≡N.
Nitroso Compounds constitute a group of compounds that contain the structural element R-N=O and have the NO group attached to an organic moiety.
Organometalics are compounds that contain a metal bonded to a carbon. The bonds may be ionic or covalent depending on the nature of the metal ion and the carbon content. Organometalics are used in industrial processes, pharmaceutical and agricultural applications, and have limited use in consumer products. Metalorganics are metal-containing compounds containing organic ligands.
Non-VOC (non-volatile-organic compound) refers to the status of the ingredient in the U.S. Environment Protection Agency National Volatile Organic Compound Emission Standards for Consumer Products (i.e., the “National Consumer Products Rule”) that is codified at 40 C.F.R. Part 59 Subpart C, as well as the California Air Resources Board “Consumer Products Regulation” that is codified at Cal. Code Regs. Title 17 §§ 94507-17, and numerous state air quality regulations based on the Ozone Transport Commission Mode Rule for Consumer Products. As used in this Dictionary, refers to organic compounds with no measurable vapor pressure at 20 degrees C.
Organic Salts is a group of salts formed by the reaction of an organic base with either an inorganic or organic acid or by the reaction of an inorganic base with an organic acid. Prominent members of this class are the salts of Carboxylic Acids. The use of salts is preferred as a rule over that of acids and bases since salts may be more stable raw materials and because salts exhibit greater water solubility. The following listing excludes the salts of amines and alkanolamines which are classified as Amines, and the salts of fatty acids, which are classified as Soaps. Also excluded are the salts of anionic and cationic surfactants, which can be found in the class listings of the functional groups responsible for surface activity.
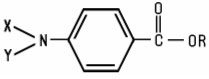
PABA Derivatives are derivatives of p-aminobenzoic acid have the following structure:
Phenols (including salts) are synthetic or natural aromatic compounds which carry at least one -OH group on an aromatic ring. Additional ring substitutions are common. The bactericidal properties of phenols are widely employed. Their antioxidant properties (e.g., Di-t-Butyl Hydroquinone or BHA) are also important. They are widely used in oxidation and as flavors (Methyl Salicylate).
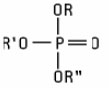
Phosphorus Compounds (including salts) used as ingredients are primarily organic esters of orthophosphoric acid which conform to the structure:
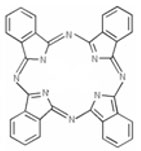
Phthalocyanine Compounds constitute a group of organic or organometallic compounds that contain the phthalocyanine structural element:
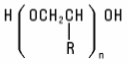
Polymeric Ethers (Homo and block) Polymeric Ethers are formed by polymerization of monomeric alkylene oxides, generally ethylene or propylene oxide. The molecular weights of these polymers may be quite low (e.g., PEG-4) or as high as several million (e.g., PEG-115M). Polyethers possess terminal hydroxyl groups, which also makes them Alcohols. They have the structure:
Polyols are compounds which contain three or more hydroxyl groups per molecule. The most important members of this class are Glycerin and Sorbitol, which are used as cosolvents.
Proteins (Including enzymes) are long-chain, high molecular weight polymers formed by the selfcondensation of Amino Acids (an amidation reaction). Also included in this class are proteins produced by recombinant technology. Naturally occurring, water-soluble proteins are somewhat unstable and tend to precipitate or denature when exposed to high temperatures or concentrated salt solutions. Water-insoluble proteins are less sensitive to denaturation, but like all proteins are subject to hydrolysis by enzymes as well as chemical reagents, such as acids or bases. Almost all enzymes are proteins which possess the ability to catalyze various chemical reactions (synthetic or hydrolytic). Typical are Urease (which produces ammonia from urea), Catalase (which produces oxygen from peroxides), and Papain (which can hydrolyze other proteins).
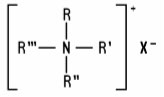
Quaternary Ammonium Compounds (including salts) (generally referred to as quats) are positively charged tetra-substituted nitrogen derivatives of the following structure:
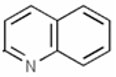
Quinoline Compounds constitute a group of heterocyclic compounds that contain the quinoline structural element:
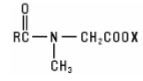
Sarcosinates and Sarcosine Derivatives are the derivatives of sarcosine, N-methyl glycine, acylated with a fatty acid chloride. They have the general structure:
Siloxanes and Silanes. Siloxanes, or organo-substituted polysiloxanes, are linear or cyclic polymers of monomeric silicon/oxygen monomers. The polymeric backbone is made up of alternating silicon and oxygen atoms. The silicon atoms may carry a wide variety of substituents which can be the same or different. The chemically least reactive substituents are the methyl or phenyl groups:
Soaps are the salts of water-insoluble Fatty Acids with various bases. Soaps are subdivided into watersoluble and water-insoluble types. The former are the salts of fatty acids with ammonia, low molecular weight Amines, especially Alkanolamines, and alkali metals, especially sodium and potassium. Waterinsoluble fatty acid salts result from reaction with metallic cations, such as zinc and aluminum, alkaline earths such as calcium and magnesium, and long-chain fatty amines. The water-soluble salts of fatty acids, derived from alkaline hydrolysis (saponification) of plant or animal Fats and Oils, are used widely in laundry and other cleaning applications.
Sulfonated Polymers are polymers containing RSO3 groups. They include the salts or esters of sulfonic acid.
Sulfonated Pyrene Compounds are aromatic organosulfur compounds that generally conform to the formula ArSO3H or ArSO3+, where Ar is the polycyclic pyrene ring.
Sulfonic Acids is a class of compounds containing a sulfur atom exhibiting a valence of +4 in the classical sense. In addition, they possess a C-S bond. The C-S bond is stable to hydrolysis, in contrast to the C-O-S bond found in Sulfuric Acid Esters. The following structure:
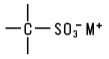
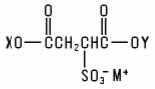
Sulfosuccinates and Sulfosuccinamates (including salts) are members of a large group of chemicals best represented as the salts of substituted sulfosuccinic acids:
Sulfuric Acid Esters (including salts; excluding alkyl sulfates and alkyl ether sulfates) is a group of compounds containing a sulfur atom exhibiting a +6 valence in the classical sense. In light of the exclusions, only a few sulfuric acid esters of substituted glycols and polyols are found in this listing. The C-O-S linkage in these materials is an ester bond and therefore subject to hydrolysis. Sulfuric acid esters find use as surface-active agents (i.e., surfactants).
Synthetic Polymers (including salts) are the large molecules prepared from relatively small chemical compounds called monomers. If only one monomer is used in the synthesis, the resulting polymer is a homopolymer. Copolymers result whenever two or more monomeric molecules are employed in the synthesis. If the monomers are blended during synthesis, random polymers result, e.g., as from mixtures of ethylene and propylene oxides. If ester or amide links are formed during polymerization, the specifics of the chemical reaction control the structure of the final polymer, e.g., Nylon. Block copolymers are prepared by first forming a homopolymer and then reacting this with a different monomer; the reactive groups of this polymer can then be further reacted with additional monomeric reagents. Cross-linked polymers are commonly formed during the synthesis of homo- or copolymers when the monomeric constituents are multifunctional. Graft Polymers are segmented copolymers with a linear backbone of one monomer and randomly distributed branches of another monomer. The molecular weight of polymers varies greatly. Polymers may be brittle solids, like Tosylamide/Formaldehyde Resin; flexible solids, like nylon or polyethylene; or liquids of varying viscosities, such as Polybutene. Polymers may be water soluble, such as PVP, or almost unaffected by any solvent, such as olychlorotrifluoroethylene. In light of the wide variety of polymers available, these materials have found uses in numerous products for a multitude of purposes. Synthetic polymers are commonly used as the primary constitutuents floor polishes and automobile polishes. Biological Polymers and their derivatives and various Waxes can also perform similar functions in various products.
Terpenes are organic compounds that conform to the formula (C5H8)n, where n is the number of linked isoprene units. The isoprene units may be linked together to form linear chains or rings.
Triphenylmethanes are organic compounds that have the basic skeleton of a trityl or triphenylmethyl group Ph3C. Triphenylmethanes can serve as pH indicators, display fluorescence, or as dyes or colorants.
Thio Compounds are derivatives of hydrogen sulfide, H2S, in which one or both of the hydrogen atoms have been replaced by an aliphatic or aromatic carbon atom or, occasionally, by a sulfur atom. If both hydrogen atoms are replaced, the compounds are thioethers (e.g., the amino acid, methionine). If only one of the hydrogen atoms is replaced by a carbon atom, the resulting compound is referred to as a thiol or a mercaptan (e.g., the amino acid, cysteine). Mild oxidation of mercaptans yields disulfides (e.g., the amino acid, cystine). Some types of thio compounds are used as antioxidants and in a variety of antibacterial and antifungal applications.
VOC (volatile organic compound) refers to the status of the ingredient in the U.S. Environment Protection Agency National Volatile Organic Compound Emission Standards for Consumer Products (i.e., the “National Consumer Products Rule”) that is codified at 40 C.F.R. Part 59 Subpart C, as well as the California Air Resources Board “Consumer Products Regulation” that is codified at Cal. Code Regs. Title 17 §§ 94507-17, and numerous state air quality regulations based on the Ozone Transport Commission Mode Rule for Consumer Products. Many ingredients in this Dictionary are mixtures containing VOC, LVP-VOC, Inorganic Compound, and/or Non-VOC content. Information on the exact percent VOC content should be obtained from the supplier.
Waxes (natural and synthetic) are a large class of organic materials that are solids at room temperature derived from a variety of sources. The word wax originally referred to relatively high melting animal or vegetable-derived lipids. In modern usage, the term wax is applied to a wide variety of chemically different lipids. Included are animal waxes, plant waxes, mineral waxes, and petroleum waxes. Microcrystalline Wax is a high molecular weight petroleum wax. Animal, plant, and some mineral waxes are primarily esters of high molecular weight fatty alcohol with high molecular weight fatty acid. For example, the hexadecanoic acid ester of tricontanol is commonly reported to be a major component of beeswax. The synthetic waxes included such diverse chemicals as polyethylene and hydrocarbon waxes derived from carbon monoxide and hydrogen (Fischer-Tropsch synthesis). Certain blends of chemicals useful for emulsification are sometimes referred to as emulsifying waxes. Waxes find uses in many types of products to impart high viscosity to emulsions and suspensions and to harden lipidbased materials.
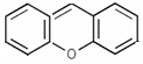
Xanthene Compounds constitute a group of heterocyclic compounds that contain the Xanthene (9Hxanthene, 10H-9-oxaanthracene) structural element: